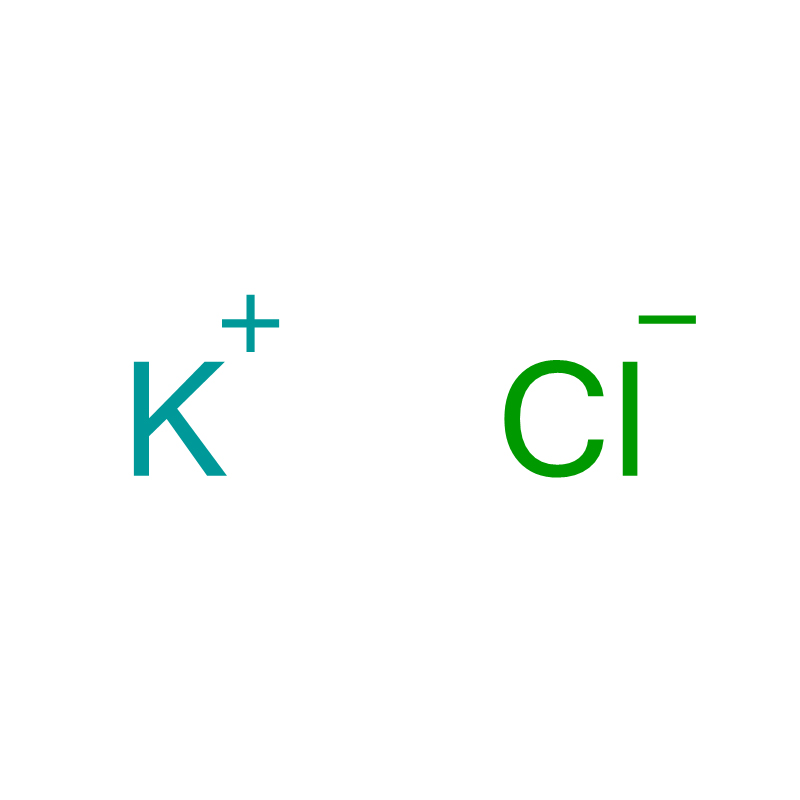Potassium Chloride Cas: 7447-40-7
| Nomoro ea lethathamo | XD91858 |
| Lebitso la Sehlahiswa | Potassium Chloride |
| CAS | 7447-40-7 |
| Foromo ea molek'hulela | ClK |
| Boima ba Molek'hule | 74.55 |
| Lintlha tsa polokelo | 2-8°C |
| Khoutu ea Lekhetho e Kopantsoeng | 31042090 |
Tlhaloso ea Sehlahisoa
| Ponahalo | Phofo e tšoeu ea kristale |
| Assay | 99% mets |
| Sebaka se qhibilihang | 770 °C (hote.) |
| Ntho e belang | 1420°C |
| tekano | 1.98 g/mL ho 25 °C (hote.) |
| index ea refractive | n20/D 1.334 |
| Fp | 1500°C |
| ho qhibiliha | H2O: e qhibilihang |
| Matla a Khoheli a khethehileng | 1.984 |
| Monko o monate | Ha e na monko |
| PH | 5.5-8.0 (20℃, 50mg/mL ho H2O) |
| PH Range | 7 |
| Ho qhibiliha ha Metsi | 340 g/L (20 ºC) |
| λmax | λ: 260 nm Boholo: 0.02 λ: 280 nm Boholo: 0.01 |
| E hloahloa | Hygroscopic |
| Sublimation | 1500 ºC |
| Botsitso | E tsitsitseng.Ha e lumellane le li-oxidizing tse matla, li-acid tse matla.Sireletsa mongobo.Hygroscopic. |
Potassium chloride (KCl) e sebelisoa litokisong tsa lithethefatsi hape e le motsoako oa lijo le reagent ea lik'hemik'hale.Hoa khoneha ho fokotsa sodium lijong tsa hau ka ho kenya potassium chloride bakeng sa letsoai la tafole (sodium chloride), e ka 'nang ea e-ba bophelo bo botle.Molten potassium chloride e boetse e sebelisoa tlhahisong ea electrolytic ea metallic potassium.KCl e boetse e fumanoa metsing a leoatle a brine 'me e ka ntšoa ho mineral carnallite.
Potassium Chloride ke limatlafatsi, tlatsetso ea lijo, le sesebelisoa sa gelling se teng e le likristale kapa phofo.e na le ho qhibiliha ha 1 g ho 2.8 ml ea metsi ka 25 ° c le 1 g ho 1.8 ml ea metsi a belang.hydrochloric acid, le sodium chloride le magnesium chloride li fokotsa ho qhibiliha ha eona metsing.e sebelisoa e le sebaka sa letsoai le tlatsetso ea liminerale.e na le tšebeliso ea boikhethelo ho jelly e tsoekere e entsoeng ka maiketsetso le ho boloka.e sebelisoa e le mohloli oa potassium bakeng sa mefuta e itseng ea li-gels tsa carrageenan.e sebelisoa ho nkela sodium chloride sebakeng sa lijo tse nang le sodium e tlase.
Potassium chloride ke reagent ea laboratori e sebelisetsoang ho eketsa viscosity ea sehlahisoa litokisetsong tsa litlolo le tsa meriana.
Potassium chloride (KCl), eo hangata e bitsoang muriate of potash, ke mohloli o atileng haholo oa potash (K2O), mme e etsa karolo ea 95% ea tlhahiso ea potash lefatšeng.Hoo e batlang e le tsohle (90%) potash ea khoebo e ntšoa mehloling ea tlhaho ea letsoai la potasiamo e hlahang libetheng tse tšesaane lijaneng tse kholo tsa letsoai tse entsoeng ke moea oa maoatle a khale.Matša a kajeno a letsoai le matsoai a tlhaho a emela hoo e ka bang 10% ea potash e ka khutlisoang.Ho ntšoa ho lateloa ke ho sila, ho hlatsoa, ho hlahloba, ho phaphamala, crystallization, ho hloekisa le ho omisa.
Ho feta 90% ea kakaretso ea tšebeliso ea KCl e sebelisetsoa tlhahiso ea manyolo.Tlhahiso ea hydroxide ea potasiamo e etsa karolo e fetang 90% ea tšebeliso e seng ea manyolo kapa ea indasteri ea KCl.KOH e boetse e sebelisoa tlhahisong ea menontsha ea metsi ea boemo ba temo.Lisebelisoa tsa KCl li kenyelletsa:
Potassium chloride (KCl) ke letsoai le sa sebetseng le sebelisetsoang ho etsa menontsha, kaha kholo ea limela tse ngata e lekanyelitsoe ke ho noa ha tsona potassium.Potasiamo ea limela e bohlokoa bakeng sa taolo ea osmotic le ionic, e phetha karolo ea bohlokoa ho homeostasis ea metsi 'me e amana haufi-ufi le mekhoa e amehang tlhahisong ea protheine.
Ka ho nka lifoto.Litharollong tsa buffer, lisele tsa electrode.
Potassium chloride e ka sebelisoa bakeng sa ho lokisa saline ea phosphate buffered saline, le bakeng sa ho ntšoa le ho kopanya liprotheine.
E sebelisoa ho litharollo tsa buffer, meriana, ts'ebeliso ea mahlale, le ho lokisa lijo.
E sebelisoa ka limatlafatsi;moemeli oa gelling;sebaka sa letsoai;tomoso lijo.
lijo/lijo tse tlatselletsang lijo: KCl e sebelisoa e le limatlafatsi le/kapa lijo tse tlatsetsang lijong.KCl e boetse e sebetsa e le tlatsetso ea potassium lijong tsa liphoofolo.
lihlahisoa tsa meriana: KCl ke moemeli oa bohlokoa oa phekolo, o sebelisoang haholo-holo ho phekola hypokalemia le maemo a amanang.Hypokalemia (khaello ea potasiamo) ke boemo bo ka 'nang ba bolaea' mele moo 'mele o hlōlehang ho boloka potasiamo e lekaneng ho boloka bophelo bo botle.
lik'hemik'hale tsa laboratori: KCl e sebelisoa liseleng tsa electrode, tharollo ea buffer, le spectroscopy.
ho cheka seretse bakeng sa indasteri ea tlhahiso ea oli: KCl e sebelisoa e le sekontiri ka har'a seretse sa ho cheka oli le joalo ka sekontiri sa shale ho thibela ho ruruha.
li-retardants tsa lelakabe le liente tse thibelang mollo: KCl e sebelisoa e le karolo ea setima-mollo sa lik'hemik'hale tse omeletseng.
li-anti-freezing agents: KCl e sebelisetsoa ho qhibilihisa leqhoa literateng le li-driveways.
Hoo e ka bang 4-5% ea tlhahiso ea potash e sebelisoa lits'ebetsong tsa indasteri (UNIDOIFDC, 1998).Ka 1996, phepelo ea lefats'e ea potash ea boemo ba indasteri e ne e le haufi le 1.35 Mt K2O.Lisebelisoa tsena tsa indasteri li hloekile ka 98-99%, ha li bapisoa le tlhahiso ea potash ea temo ea 60% K2O bonyane (e lekanang le 95% KCl).Potash ya indasteri e lokela ho ba le bonyane 62% K2O mme e be le maemo a tlase haholo a Na, Mg, Ca, SO4 le Br.Potash ena ea boemo bo holimo e hlahisoa ke bahlahisi ba fokolang feela lefatšeng ka bophara.
Potassium hydroxide (KOH), eo hape e tsejoang e le caustic potash, ke sehlahisoa se seholo ka ho fetisisa sa K bakeng sa tšebeliso e sa sebeliseng manyolo.E hlahisoa ke electrolysis ea indasteri ea KCl 'me e sebelisoa haholo bakeng sa ho etsa sesepa, li-detergents, mafura, li-catalysts, rabara ea maiketsetso, lipapali, lidae le likokoanyana tse bolaeang likokoanyana.Caustic potash e boetse e le manyolo a metsi hape e le motsoako oa libeteri tsa alkaline le lik'hemik'hale tse sebetsanang le lifilimi.
Potassium hydroxide ke ntho e tala tlhahisong ea matsoai a fapaneng a K, haholo-holo K carbonates, hape le citrate, silicates, acetates, joalo-joalo , li-chinaware le li-tubes tsa TV.Potassium bicarbonate e sebelisoa haholo indastering ea lijo le ea meriana.
Lik'hemik'hale tse entsoeng ka potash le letsoai li boetse li sebelisoa ha ho etsoa lihlahisoa tsa tšepe, nama e phekotsoeng, tšepe e halefileng, li-fumigants tsa pampiri, tšepe e thata, li-bleaching agents, phofo e bakang, tranelate ea tartar le lino tse tahang.Lefatšeng ka bophara, KCl ea indasteri e hakanngoa hore e tla sebelisoa ka tsela e latelang: li-detergents le sesepa, 30-35%;khalase le ceramics, 25-28%;masela le dae 20-22%;lik'hemik'hale le lithethefatsi, 13-15%;le lisebelisoa tse ling, 7-5% (UNIDO-IFDC, 1998).
Potassium chloride ke reagent e sebelisoang haholo ho biochemistry le biology ea limolek'hule.Ke karolo ea phosphate buffered saline (PBS, Product No. P 3813) le ea polymerase chain reaction (PCR) buffer (50 mM KCl).
KCl e boetse e sebelisoa lithutong tsa lipalangoang tsa ion le likanale tsa potasiamo.
KCl e boetse e sebelisoa ho solubilization, ho ntšoa, ho hloekisa le ho kopanya liprotheine.
Tšebeliso ea KCl ho crystallization ea histone core octamers e tlalehiloe.